Abstract
Introduction : In the past decade, the combination of high-dose melphalan (MEL) and auto-hematopoietic stem cell transplantation (auto-HCT) with novel agents substantially improved the outcomes in younger patients with multiple myeloma. However, the safety and efficacy of auto-HCT in patients aged ≥65 years remain uncertain. Large clinical trials evaluating the role of auto-HCT in multiple myeloma mostly included patients aged <65 years even in the era of novel agents. Here, we examined the safety and efficacy of auto-HCT in patients with multiple myeloma who were aged ≥65 years.
Methods: We examined 2,056 patients aged ≥16 years who underwent auto-HCT for multiple myeloma from 2007 to 2014; they were selected based on the following criteria: (1) first auto-HCT with peripheral blood stem cells; (2) use of MEL alone (100, 140, and 200 mg/m2) as a conditioning regimen; and (3) without planned tandem transplantation. A total of 2,056 patients met these criteria, and 287 of them were aged ≥65 years. The era of novel agents was defined as the date of transplantation after December 2006 because bortezomib was approved for public administration in Japan in December 2006. The primary end-point was 100-day treatment-related mortality (TRM), i.e., not myeloma-related or accidental deaths within 100 days after the first auto-HCT, and the secondary end-point was overall survival (OS). To adjust for a selection bias, the 100-day TRM was compared between two age groups (<65 vs. ≥65 years) by a propensity score analysis with the following factors: sex, immunoglobulin subtype, Durie-Salmon staging system and international staging system (ISS) stages at diagnosis, renal impairment at diagnosis, cytogenetic abnormalities, disease status at transplantation, conditioning regimen, and performance status (PS) at transplantation. Finally, 1:1 matched pairs were extracted. The probability of 100-day TRM was calculated using the Gray test and assessed by cumulative incidence analysis. The probability of OS was estimated using the Kaplan-Meier method and compared between groups using the log-rank test. Multivariate analysis for OS was performed using the Cox proportional hazards model.
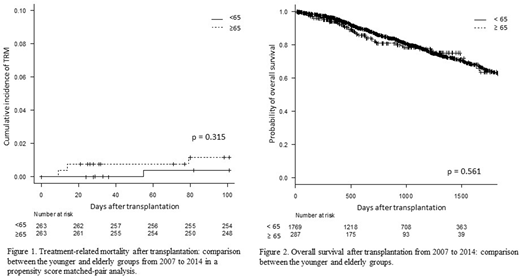
Results: The median age at transplantation was 66 (range, 65-76) and 57 (range, 18-64) years in the elderly and younger groups, respectively. The number of patients who used 100, 140, and 200 mg/m2 MEL were 17 (1.0%), 71 (4.0%), and 1,681 (95.0%), respectively, in the younger group and 19 (6.6%), 51 (17.8%), and 217 (75.6%), respectively, in the elderly group, with a significant difference (p<0.001). The number of 100-day TRM and deaths due to disease relapse or progression were 13 (0.7%) and 5 (0.3%), respectively, in the younger group and 3 (1.0%) and 1 (0.3%), respectively, in the elderly group. A matched-pair analysis was performed based on the propensity score, and 263 patients were extracted from each group. The 100-day TRM probability was 0.4% (95% confidence interval [CI]: 0.0-2.0%) and 1.2% (95% CI: 0.3-3.1%) in the younger and elderly groups, respectively, without significant difference (p=0.315, Figure 1) in the propensity score-matched pair analysis. The probabilities of 5-year OS after transplantation were 62.5% (95% CI: 58.6-66.1%) and 63.5% (95% CI: 52.2-72.7%) in the younger and elderly groups, respectively, without significant difference (p=0.561, Figure 2). In the multivariate analysis, except age at transplantation (<65 or ≥65 years), gender, immunoglobulin subtype, ISS stage, unfavorable cytogenetic abnormalities, disease status at transplantation, and PS were significantly associated with OS.
Conclusion: The 100-day TRM and OS were not significantly different between the younger and elderly patients who underwent auto-HCT for multiple myeloma in 2007-2014. We showed that auto-HCT is safe and effective for treating multiple myeloma in elderly patients, particularly in the era of novel agents. A comparable benefit was observed in elderly patients who underwent auto-HCT for multiple myeloma, highlighting the fact that chronologic age alone should not be used to determine transplantation eligibility.
Hanamura:CHUGAI PHARMACEUTICAL CO., LTD.: Research Funding; Kyowa Hakko Kirin Company, Limited: Research Funding; Bristol-Myers Squibb: Other: Lecture fee, Research Funding; Celgene: Other: Lecture fee; Takeda Pharmaceutical Company Limited.: Other: Lecture fee; Fujimoto Pharmaceutical Corporation: Research Funding. Sunami:Sanofi: Research Funding; AbbVie: Research Funding; Daiichi-Sankyo: Research Funding; MSD: Research Funding; Takeda: Research Funding; Bristol-Myers Squibb K.K.: Honoraria, Research Funding; GlaxoSmithKline: Research Funding; Ono: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Research Funding; Janssen: Research Funding. Mori:Janssen: Honoraria; Eisai: Honoraria; Japan Blood Products Organization: Honoraria; Shire Japan: Honoraria; Kyowa Hakko Kirin: Honoraria; Ono: Honoraria; Celgene: Honoraria; MSD: Honoraria; Taisho Toyama Pharmaceutical Co: Honoraria; Pfizer: Honoraria; Novartis Pharma: Research Funding; Asahi Kasei: Research Funding; Astella Pharma: Honoraria; CHUGAI: Honoraria; SHIONOGI: Honoraria; Novartis Pharma: Honoraria; MSD: Research Funding. Iida:MSD: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Ono: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Kyowa-Hakko Kirin: Research Funding; Gilead: Research Funding; Sanofi: Consultancy; Teijin Pharma: Research Funding; Toyama Chemical: Research Funding; Astellas: Research Funding; Chugai: Research Funding. Kako:Takeda Pharmaceutical Company Limited.: Honoraria; Takeda Pharmaceutical Company Limited.: Honoraria; Celgene K.K.: Honoraria; Bristol-Myers Squibb: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Ono Pharmaceutical Co., Ltd.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria. Sawa:Celgene Corporation: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis International AG: Honoraria; CHUGAI PHARMACEUTICAL CO., LTD.: Honoraria; Mundipharma K.K.: Honoraria. Kanda:Dainippon-Sumitomo: Consultancy, Honoraria, Research Funding; Eisai: Consultancy, Honoraria, Research Funding; Chugai: Consultancy, Honoraria, Research Funding; Otsuka: Research Funding; Nippon-Shinyaku: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Kyowa-Hakko Kirin: Consultancy, Honoraria, Research Funding; Taiho: Research Funding; Pfizer: Research Funding; MSD: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Asahi-Kasei: Research Funding; Ono: Consultancy, Honoraria, Research Funding; Sanofi: Research Funding; Novartis: Research Funding; Shionogi: Consultancy, Honoraria, Research Funding; Taisho-Toyama: Research Funding; CSL Behring: Research Funding; Tanabe-Mitsubishi: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Mochida: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Takara-bio: Consultancy, Honoraria. Ichinohe:Celgene: Honoraria; JCR Pharmaceuticals: Honoraria; Bristol-Myers Squibb: Honoraria; Alexion Pharmaceuticals: Honoraria; Zenyaku Kogyo Co.: Research Funding; Janssen Pharmaceutical K.K.: Honoraria; Mundipharma: Honoraria; Takeda Pharmaceutical Co.: Research Funding; Taiho Pharmaceutical Co.: Research Funding; Sumitomo Dainippon Pharma Co.: Research Funding; Repertoire Genesis Inc.: Research Funding; Otsuka Pharmaceutical Co.: Research Funding; MSD: Research Funding; Nippon Shinyaku Co.: Research Funding; Pfizer: Research Funding; Ono Pharmaceutical Co.: Research Funding; Kyowa Hakko Kirin Co.: Research Funding; Eisai Co.: Research Funding; CSL Behring: Research Funding; Chugai Pharmaceutical Co.: Research Funding; Astellas Pharma: Research Funding; Novartis.: Honoraria. Takamatsu:Bristol-Myers Squibb: Research Funding; Ono: Research Funding; Janssen: Honoraria; Celgene: Honoraria, Research Funding. Takami:Chugai: Research Funding; Bristol-Myers Squibb: Research Funding; Kyowa Hakko Kirin: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal